Electron affinity is the amount of energy released when an electron is added to the valence shell of an isolated gaseous atom.
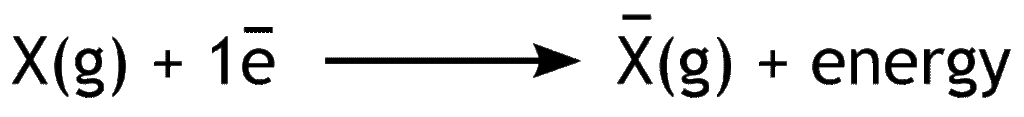
Electron affinity shows the measure of ability of a neutral atom to gain electron. Depending on the number of added electrons, there may be first electron affinity, second electron affinity, third electron affinity and so on.
The first electron affinity is negative, second and third electron affinities are positive. The positive values of second and third electron affinities is because electron is added to anions which repel the adding electron (like charges repel each other). To overcome this repulsion, energy is required, which makes the process endothermic and the electron affinities positive.
The electron affinities of non-metal atoms are negative (exothermic) because these atoms attract electrons to complete their outer shells, and this attraction results in the release of energy. However, the electron affinities of metals are positive (endothermic) because they do not need electrons, rather they want to lose electrons. When electron is added to the valence shell of an isolated metal atom, energy is required (endothermic process) because metal atoms repel the adding electrons.
Periodic Trends
Across a period from left to right, the electron affinities of elements increase, which means they become more negative.