Introduction
Atomic structure builds the foundation of chemistry because it explains the composition, behaviour, and interactions of matter at the smallest level. All substances, living or non-living, are made up of atoms—the basic units of matter. Understanding the structure of atom helps explain chemical reactions, bonding, periodic trends, and the properties of elements.
The concept of the atom has developed over centuries. In 1808, John Dalton proposed his Atomic Theory, describing the atom as indivisible and the smallest unit of matter. Discharge tube experiments in the late 19th and early 20th centuries revealed the existence of electrons, protons, and neutrons.
The first atomic model was proposed by J J Thomson in 1904 after he discovered electron in 1897. His atomic model is called Plum Pudding model (plum pudding is English dessert) . He stated that atom is a spherical particle where the positive charge is uniformly distributed and electrons embedded within it just like plums in pudding or seeds in watermelon (seeds electron while the red part is positive charge).
In 1911, Ernest Rutherford introduced the nuclear model, showing that most of the atom’s mass and positive charge is concentrated in a tiny nucleus, with electrons revolving around it.
In 1913, Niels Bohr improved this model by proposing quantized energy levels (shells) for electrons. He calculated the atomic radius of hydrogen atom and the energy of its electrons. He also explained its atomic emission spectrum where each spectral line represented the energy levels (shells or orbits).
Powerful spectrometers later revealed that the spectral lines of hydrogen atom split into finer lines, proving the existence of sub-shells and orbitals.
In 1926, Erwin Schrödinger introduced the wave mechanical model, describing electrons as waves, not particles. He added that electrons have maximum probability in some regions called orbitals.
In 1927, Werner Heisenberg formulated the Uncertainty Principle, stating that the exact position and momentum of an electron cannot be known simultaneously, rejecting the idea of fixed orbits of Niels Bohr.
Sub-Atomic Particles and Fundamental Properties
Atoms are made of three tiny particles called sub-atomic particles: electrons, protons and neutrons. Each of these particles has different charge, mass, and position inside the atom.
Position in the Atom
- Electrons move around the nucleus in energy levels or shells.
- Protons and neutrons are found in the nucleus.
Relative Charges
Relative charge is the ratio of the charge on a particle compared to the fundamental charge. Fundamental charge is the charge equal in magnitude to the charge on proton or electron (1.6 x 10-19 Coulomb).
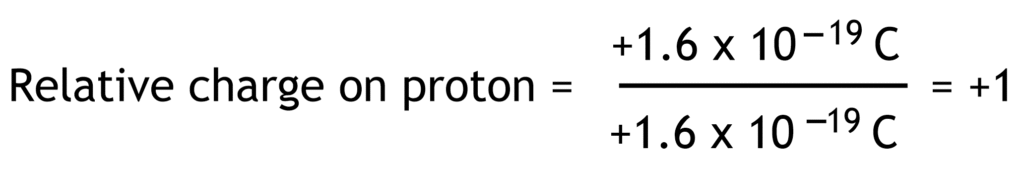

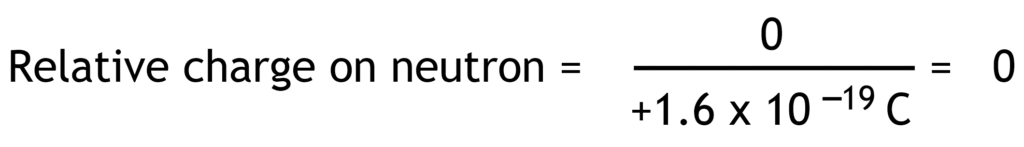
Relative charges of electron, proton and neutron have no units because they are ratios.
Relative Masses
Relative mass of a particle is the ratio of its actual mass to one atomic mass unit.
1 atomic mass unit (u) = 1.660 539 x 10-27 kg
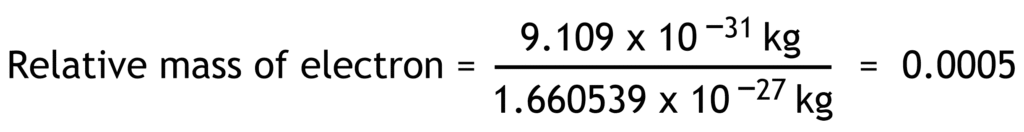
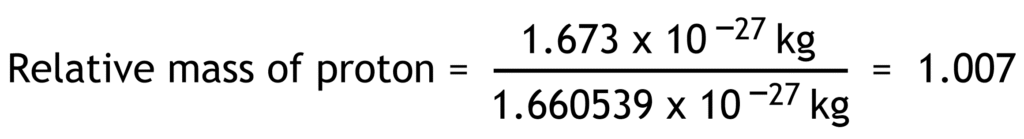
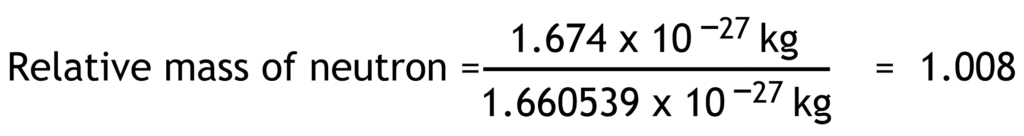
| Particle | Symbol | Fundamental charge (C) | Relative charge | Actual mass (kg) | Relative mass (u) |
| proton | p+ | +1.602 x10-19 | +1 | 1.673 x10-27 | 1.007 |
| neutron | n0 | 0 | 0 | 1.674 x10-27 | 1.008 |
| electron | e– | -1.602 x10-19 | -1 | 9.109 x10-31 | 0.0005 |
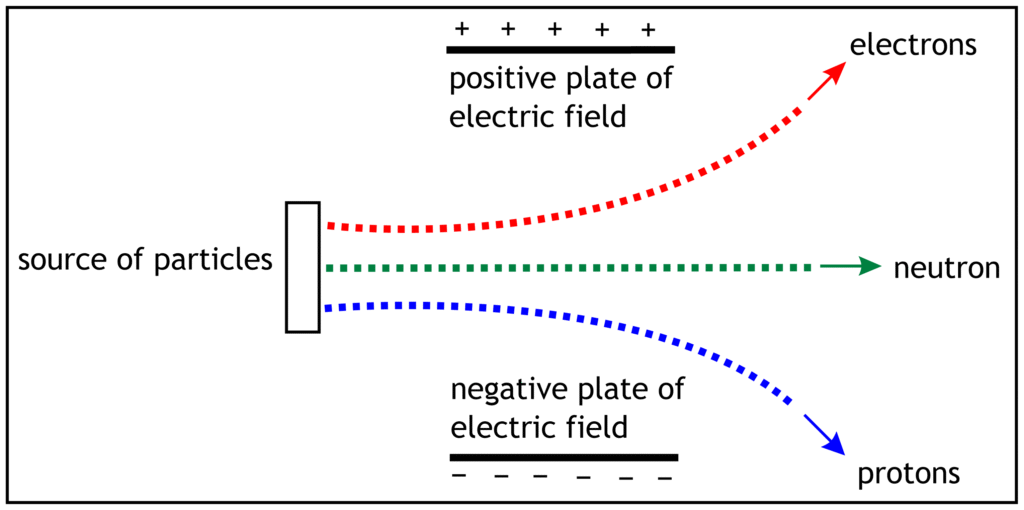
Effect of Electric Field on Sub-Atomic Particles
The behaviour of electron, proton and neutron depend on their charges and masses. When a beam of electrons, protons and neutrons is passed with same speed through electric field, they show the following pattern.
Electron move in the direction of positive plate of electric field which proves that they carry negative charge. Protons travel in the direction of negative plate of electric field. This confirms that protons possess positive charge. Neutrons do not carry any electric charge, so they do not move in any directions. The travel in straight line.